
For PATIENTS – OVERVIEW
What is GuARDS?
GuARDS is a research study running in Intensive Care Units (ICU) across the UK. Patients with Acute Respiratory Distress Syndrome (ARDS) and the treatment being tested is a safe drug called dexamethasone alongside the standard ICU care.
What is Acute Respiratory Distress Syndrome (ARDS)?
Acute Respiratory Distress Syndrome is a life threatening condition where the lungs cannot provide the body’s vital organs with enough oxygen. It is usually a complication of a serious health condition meaning people are already in hospital when they develop ARDS. Patients are treated within intensive care units and will have support from machines to help them breathe. ARDS is common with 1 in 4 adults in intensive care units developing the condition. All age groups are affected with high mortality rates. Patients who survive and leave intensive care have a long-term reduction in their quality of life and often attend their doctor or hospital more often than they did before.
What causes ARDS?
ARDS happens when the lungs become severely inflamed from an infection or injury. The inflammation causes fluid from nearby blood vessels to leak into the tiny air sacs in your lungs, making breathing increasingly difficult.
The lungs can become inflamed after:

Who can take part?
Patients admitted to critical care units in the UK with a condition called ARDS can participate in the trial. If you are or have recently been in an ICU in the UK, or have a relative who has, then you may have been told about GuARDS. The doctors and nurses know who can take part and will speak to you or your relative if they think you, or they, are eligible to take part.
Patients themselves or their nominated relative or medical professional independent of the study can give consent for participation on behalf of the patient.
What type of trial is GuARDS?
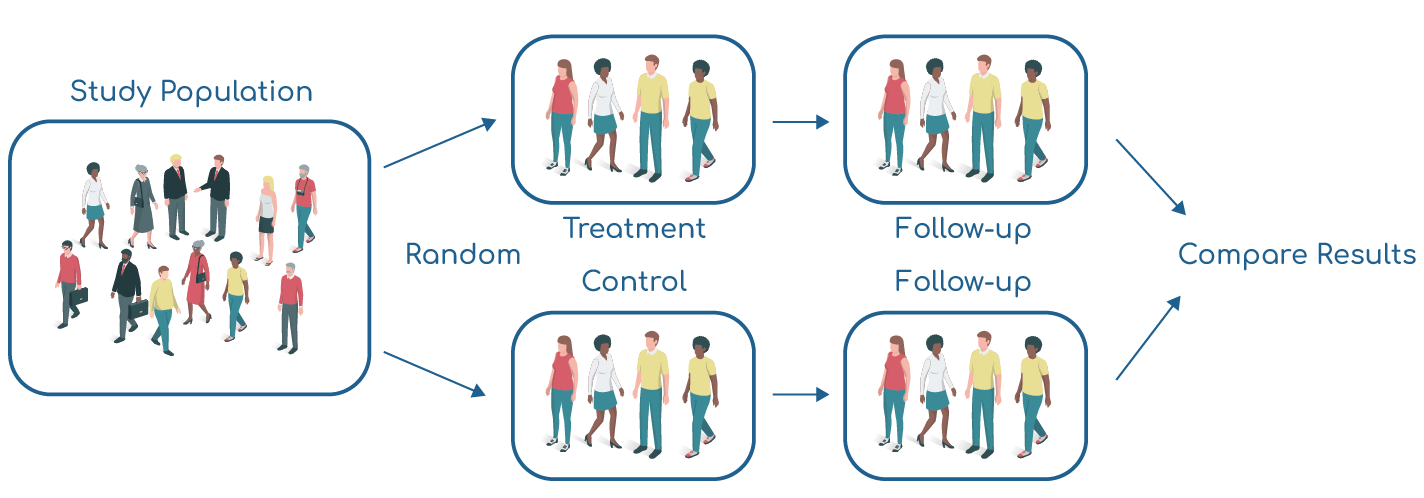
GuARDS will use a study design known as Randomised Controlled Trial (RCT).
Participants are randomly assigned to one of two groups. For GuARDS the groups are:
- Usual Care or
- Usual Care + Dexamethasone Treatment
Patients are randomly assigned by a computer to reduce any bias in the groups.
GuARDS will be an “open-label” trial where patients and the medical team looking after them will know what group they have been assigned to.
What is the outcome of the study?
Two out of five patients with ARDS do not survive beyond 60 days. We are assessing whether treatment with dexamethasone reduces this risk of dying in patients with ARDS.
We will also collect other information from patients medical notes and during our follow-up questionnaires to assess whether dexamethasone treatment is cost effective for the NHS in these patients.
What will happen to patients if they take part in the study?
This is an overview of how a patient will move through the trial:
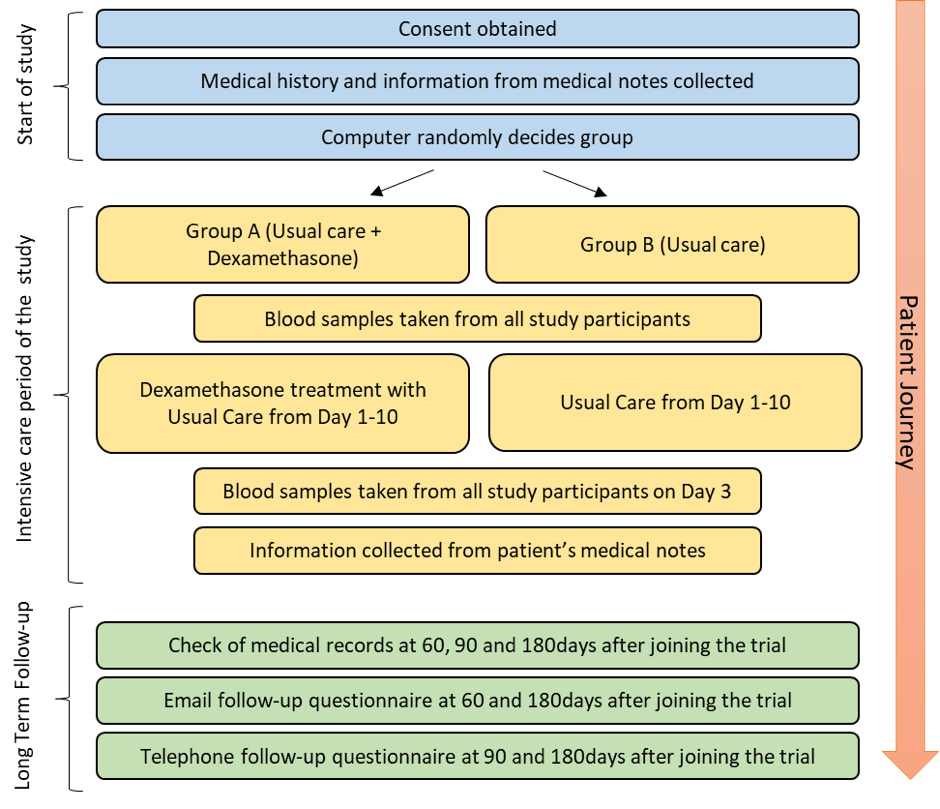
The doctors and nurses in ICU will identify what patients can take part in GuARDS. If you, or your relative, might be eligible to take part, a doctor or nurse will speak to you about how it will work.
They will take written consent for you to take part in the trial. They will then collect information from the medical notes and a computer will randomise each patient to either Usual Care or Usual care + Dexamethasone.
We will take blood samples after patients are randomised and again around day 3 of the trial for research. This will allow us to see how the dexamethasone is working.
Patients will be contacted around 60, 90 and 180 days after starting the trial with some follow-up questions around how they are doing and whether they have visited their doctor or GP since leaving hospital.
For PATIENTS – Contact
I am already in GuARDS but want to talk to someone about this?
If you are already in GuARDS but have questions or want to discuss this with someone, you should get in touch with the GuARDS contact listed on the information sheet you were given when you signed the consent form.
If you have lost this sheet or would prefer to discuss this with someone else, please contact your ICU doctor or nurse. It is important that you talk to the team at your own hospital as they will have access to the relevant information on your participation in the trial..
eMail GuARDS
If you are still finding it difficult to speak to someone about GuARDS then you can email the trial inbox guards@ed.ac.uk.
*Please note this is a University mailbox, manned by administrative staff only. Therefore please do not include any personal or sensitive information in your initial email.
Find Out More
Find out more about the trial
For more details about the trial contact: guards@ed.ac.uk
Or fill out the form